Integration of multi-omics with clinical trials data
Discover how the Genestack ODM (ODM) clinical-omics integration module - an extension of Genestack’s flagship ODM product - allows the integration of harmonised and unified representations of clinical and Life Science Data within a single user interface.
Setting out the need
As the industry moves and looks for ways to bring about savings and efficiencies into the current state of clinical drug development - with the average cost of bringing a drug to the market currently hovering around the $2.6B mark - the use of precision medicine techniques using Life Science Data holds the promise of making a small dent into these costs.

Looking within
A start has to be made somewhere, and where better for sponsors to look than what’s in house?
Since the mid 2000s, when sequencing of the human genome became readily accessible to industry, sponsors have judiciously included within their clinical studies an informed consent to include the DNA sequencing information of trial subjects. So while the sequencing information was not specifically a “protocol requirement”, and therefore not part of submission data, the “clinical data sets”, or of the IND submission, this wealth of Life Science Data already exists within many sponsor organisations.
Our estimates indicate a top 25 pharma would have anything from 2,000 to 5,000 completed studies over the last 15 years with associated Life Science Data.
This conservatively translates to anywhere between 400K and 600K subjects across the broad therapeutic range.
The opportunity
In a recent paper about omics-based strategies in precision medicine published in the International Journal of Molecular Sciences1, the authors state:
“Precision medicine capitalizes on two conceptual and technological advancements and stands on two main pillars: data generation and data modeling. Furthermore, bioinformatics has enabled comprehensive multi-omics and clinical data integration for insightful interpretation. Despite their promise, the translation of these technologies into clinically actionable tools has been slow.”
The existence of the high quality private retrospective data within a sponsor’s environment represents a whole new opportunity on the path towards precision medicine techniques by mining genome sequencing data and related clinical data within a single unified data catalogue.
The benefits of such an association are multifold - consider for example: the discovery of omics-based biomarkers - for predictive use in subject selection or patient profiling; and efficacy determinations as inclusion / exclusion criteria within planned / future clinical trials.
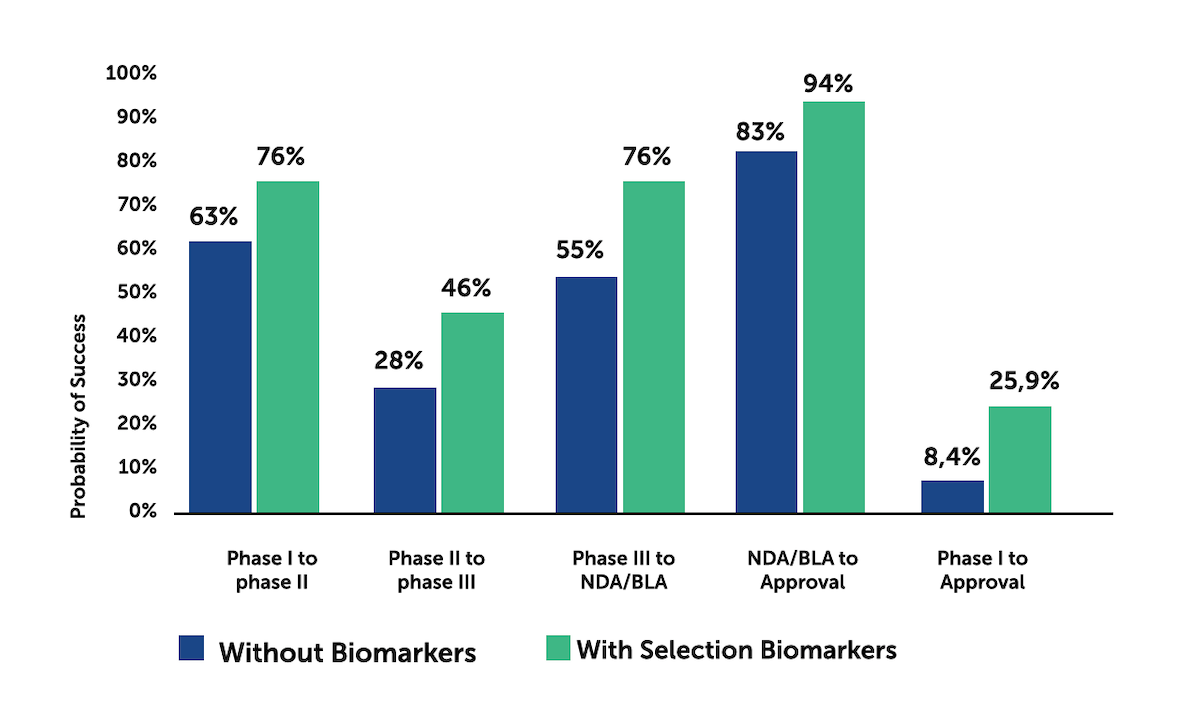
It has been recognised that selection of biomarkers strongly correlates with a higher probability of success of clinical trials. In the past, due to the unavailability of data, omics based biomarkers were underutilized and present in less than 5% of trials worldwide2.
Published studies and related data have also shown that the probability of success for clinical trials that make use of biomarkers is significantly greater2.
Based upon data gathered over a period of ten years there is a LOA three times greater than that of trials conducted without the use of biomarkers: 25.9% against 8.4% for Phase I-to-Approval (figure 1).
But there is a problem
As always there is a problem. This arises from the fact that since sequencing information was never part of “submission data” it was never intended nor designed to be associated with the repository of clinical data from completed studies.
As a consequence these data assets reside within different organisational systems (eg.sequencing systems, entimICE, etc) and not considered as a part clinical data submissions or “trial data”.
To compound this further, the disparate data types are never “harmonized” and therefore cannot be linked usefully.
The solution: Genestack's Genestack ODM
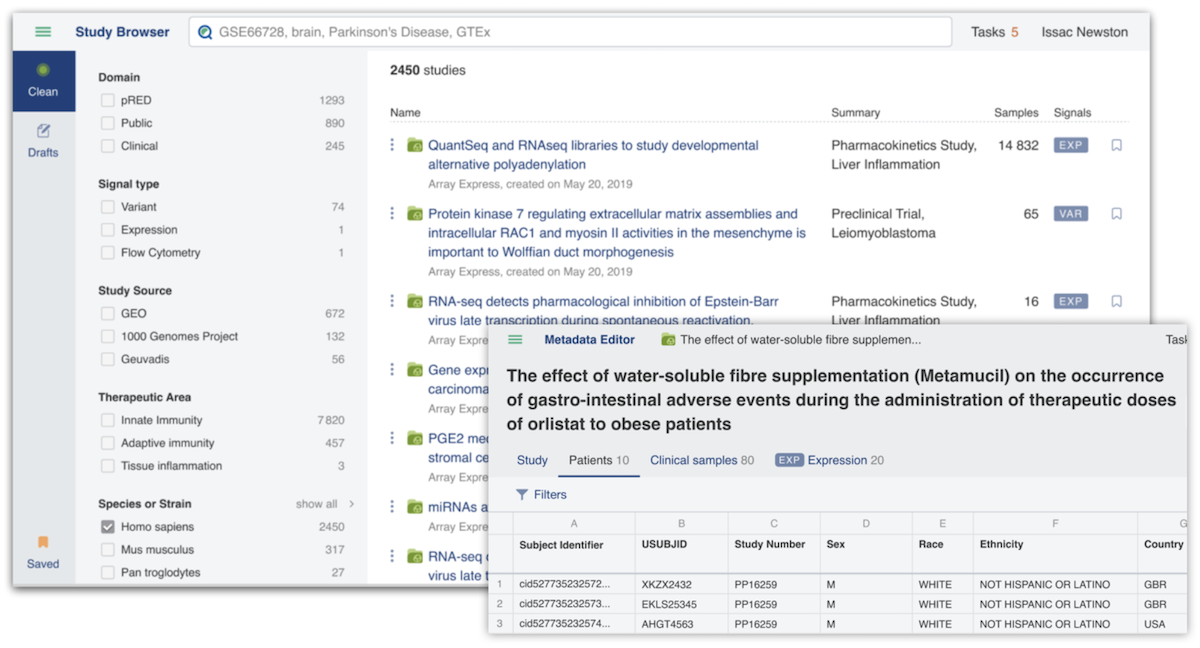
Genestack’s Genestack ODM (ODM) product allows data scientists to find, access, curate and integrate data from multiple omics sources of truth, for example genomics and transcriptLife Science Data. It achieves this through a single point of access GUI and integrative APIs for data mining. ODM has one more capability on top of being a “source of truth” - that is being the driver for making data FAIR (Findable, Accessible, Interoperable and Reusable) through its powerful curation tools, and achieving harmonisation across multiple data types.
The ODM Clinical Data Integration Module
The ODM clinical-omics integration module is an extension of Genestack’s flagship ODM product, and allows the integration of harmonised and unified representations of clinical and Life Science Data within a single user interface.
Consider the following use case:
“Across all of the organisation's non-small-cell lung carcinoma (NSCLC) studies, is there Whole Genome Sequencing data for patients with NSCLC who had no history of smoking?”
Genestack’s proprietary integration service incorporates a harmonised vocabulary key library for aggregated search across both omics and clinical data. This allows a data scientist to run a cross-study, cross-domain, search such as the use case described above to occur through a single unified representation within minutes if not seconds.
Cross-domain linkage APIs endpoints also provide and assist bulk integration between clinical and Life Science Data at scale.
To solve the problem of performant searching for users, a process which relies on data indexing, while allowing curator users to work on draft versions of data, which normal users must not be able to touch, a multiple-index system has been implemented which switches index based on the role being performed. Most users access the data in a read-only fashion and this is powered by a dedicated “published” data index, which also consumes data from clinical single sources of truth.
Do you see similar challenges within your organization?
Would you like to understand how the ODM clinical data integration module can allow you to better leverage your data assets for retrospective research? Speak to our experts and see how Genestack can make a difference to the way you manage and handle Life Science Data in your organisation.
References
1. Omics-Based Strategies in Precision Medicine: Toward a Paradigm Shift in Inborn Errors of Metabolism Investigations.Tebani et al. Int J Mol Sci. 2016 Sep 14;17(9):1555.
2. Clinical Development Success Rates 2006-2015; Bio/Biomedtracker/Amplion